TOF mass spectrometry
In many ToF-MS units, cronologic TDCs or ADCs are used to measure precisely the arrival of single ions. From the arrival time, the ion’s time-of-flight is deduced, from which the mass-to-charge ratio of the detected particle can be determined. A crucial factor for a successful measurement is the extremely low cycle-to-cycle jitter of our TDCs and their very low multiple hit detection dead time.
When it comes to finding out the composition of unknown substances, mass spectrometry is used in most cases. This applies to the checking of chemical compounds during their production and to the analysis. For this purpose, the mass of individual atoms or molecules of a sample is precisely determined. ToF mass spectrometry (ToF-MS) offers advantages in terms of accuracy and sample throughput in automated measurement methods, e.g. for high-throughput screening tools. Modern mass spectrometers often work in several stages (tandem MS, MS / MS). Particularly precise are measurements in which isolated ions are examined with a subsequent ToF measurement.
The field of ToF mass spectrometry comprises a large number of sophisticated measurement schemes that have been optimized for their respective area of application. In many analyzers, for example, the actual ToF measurement is preceded by a coupled chromatographic separation of the sample. The ToF-MS is coupled with gas chromatography (GC-MS), liquid chromatography (LC-MS), ion mobility spectrometry (IMS-MS), or with capillary electrophoresis (CE-MS) or a combination of such separation approaches. This initial separation is particularly helpful when evaluating very complex samples, e.g. in food analysis. In all of these cases, however, at the heart of the devices and the key to their precision is ultimately the time-of-flight analyzer.
Function & measurement setup:
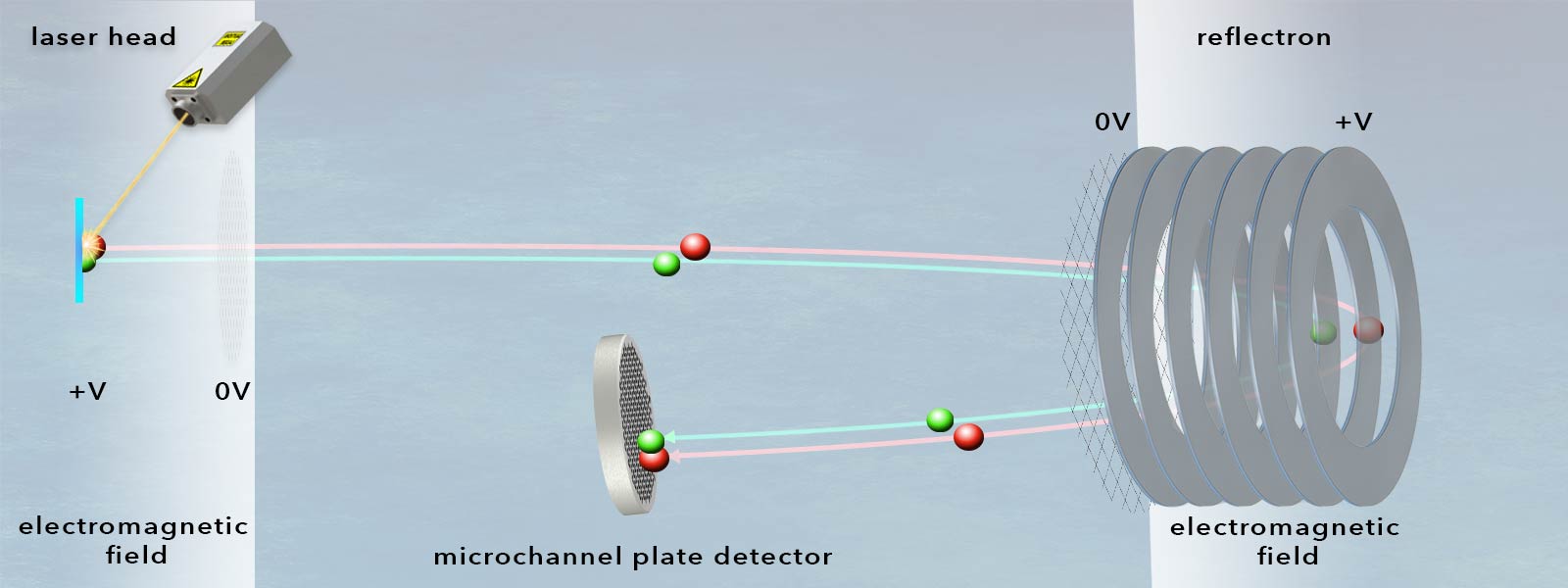
In a time-of-flight mass spectrometer, ionized atoms or molecules of a sample are separated on the basis of their mass-to-charge ratio during the passage of a flight tube, so that ultimately their mass can be determined from a measurement of their time of flight. When entering the analyzer (which consists conceptually of an electric field with known strength), the ions obtain kinetic energy proportional to their charge. After the passage of the electric field region, all ions have the same kinetic energy. However, their flight times depend on their mass such that light ions are detected earlier than heavy ions. This is the case with ions of the same charge. The measured time of flight (ToF) is recorded and is proportional to the square root of the mass of the molecule.
On the most basic level, time-of-flight analyzers essentially consist of a tube that contains a high vacuum and is equipped with an extremely fast ion detector at its end.
How is ionization of the sample implemented?
Depending on the application and the chemical, biological or physical properties of the sample to be examined, various methods of ionization can be considered. For example, if the sample is not already in the gas phase for GC-MS, it must first be vaporized or nebulized.
The sample substance is then typically ionized by particular collision (electron impact ionization), protonation (chemical ionization), or by means of strong laser light. Ideally, this process yields singly charged ions.
Here is a brief overview of various ion sources:
- Electron ionization (EI, formerly known as „electron impact ionization“ and electron bombardment ionization) is the classic type of ionization in which high-energy electrons interact with atoms or molecules in a solid or gaseous phase. An electron gun generates electrons using a hot cathode and shoots these electrons at the sample, from which ions are directly produced. The molecules to be ionized must first be vaporized by the application of thermal energy. The ions produced in this way can have different masses, but are mostly equally charged, although their excitation energy often far exceeds their ionization potential. EI transfers a large amount of oscillation energy to the sample, which leads to extensive fragmentation. This method of hard ionization is useful for analyzing volatile, thermally stable, low-mass organic compounds.
- The MALDI-TOF (matrix-assisted laser-desorption-ionization) method is very common in this regard. This uses laser light in conjunction with a chemical matrix to ionize the sample. The advantage of MALDI-TOF MS lies in the high throughput with which a wide variety of samples can be measured. The disadvantage here, however, is that the ionization efficiency varies depending on the type of analyte and is sometimes decreases rapidly. Plus the laser source can possibly be harmful to light-sensitive samples.
- The milder ionization conditions of electro-spray ionization (ESI) offer the possibility of examining unstable and light-sensitive samples more gently. To do this, the sample is dissolved in a very volatile liquid (water, ethanol, methanol). This analyte solution is then pressed through very fine metal capillaries at very high pressure so that an aerosol is formed. The tip of the needle is biased with a positive high voltage. When leaving the needle, the droplets of the aerosol absorb hydrogen ions from the protic solution. The remaining solution evaporates and the ionized droplets are attracted to the negatively biased part of the measuring device and thus accelerated.
Please note: The mass-to-charge ratio of the sample is changed by this type of ionization. When examining the time of flight, the mass of the additionally absorbed protons must be taken into account. More precisely, there are several positive charge states for each constituent of the analyte solution, so that it appears several times in the mass spectrum. The analysis of the measured data is, therefore, more complex.
- Photoionization is based on ionization through the photoelectric effect. The first laser-induced photoionization in the gas phase was observed as early as 1970. This process was later called multi-photon ionization (MPI). Organic molecules allow typically for a resonant MPI, so-called resonance-enhanced multi-photon ionization (REMPI). Today, there is extensive research on the use of photoionization in mass spectroscopy, e.g. for the investigation of nanoparticles produced by combustion. In Atmospheric Pressure Photoionization (APPI), for example, the initial ionization occurs through photoionization, whereby a light-absorbing dopant is added to the sample flow. Since comparatively few ions are generated during photoionization, this technique is considered so soft that it is ideal for studying organic molecules. This sampling technique is ideally suited for combination with a time-of-flight mass spectrometer.
- Via field ionization, strong electric fields in the immediate vicinity of sharp pins/tips, thin wires, or sharp edges may also cause ionization. The ionization takes place on the surface of the field emitter on which an electronically highly excited atom or molecule spontaneously loses an electron without any further interaction. It is therefore a very gentle ionization method (known as “field desorption”) gaining importance nowadays again, especially in combination with very accurate TOF measurements.
- Fast atom bombardment (FBA) involves shooting an accelerated beam of atoms or ions at a small metal target from an atom or ion gun. The metal target is mounted on a probe and loaded with a viscous liquid matrix (usually 3-Mercaptopropane-1,2-diol or m-NBA) in which the sample to be analyzed is dissolved. When the atom or ion beam collides with the matrix, many surface molecules of the sample are ionized and sputtered into the high vacuum. Compared to electron impact ionization, FAB is a “gentle” ionization process that is suitable for a large number of compounds, provided the sample is soluble in the matrix.
- In inductively conducted plasma mass spectroscopy (ICP-MS), a high-frequency electromagnetic field is used to generate plasma in an argon gas flow, in which a sample aerosol is heated to temperatures up to 10,000 ° C. In this process, the sample components are vaporized, broken down into their atomic components, and ionized. Coupled with TOF measurements, this ionization method offers a very good dynamic range and is considered a robust method in inorganic element analysis for element trace analysis of heavy metals such as mercury, lead, or cadmium.
Since extensive further developments are taking place with regard to ionization techniques, this list does not claim to be exhaustive.
Which detectors are used?
The separated ionic components are characterized by measuring their time of arrival at the end of the flight path using a detector. Several types of detectors are available for mass spectrometers.
- In principle, a Faraday cup is the simplest form of detector. It collects ions and becomes electrically charged in the process. It is held at a constant potential and the ions are deflected by the electric field, with the charge flowing out via a resistor and the measured voltage drop being measured and registered. The advantages of the Faraday catcher are its reliability and the possibility of measuring the ion or electron current directly as an absolute value. This system is used almost exclusively only in isotope mass spectrometry because of its low detection sensitivity. The amplification factor is 1.
- Classic photomultiplier-tubes (photoelectric multipliers, photomultipliers, PMTs) work in the ultraviolet, visible and near-infrared range of the electromagnetic spectrum. They are electron tubes that detect weak light signals (down to single photons) and amplify them to such an extent that further free electrons of lower energy are created from free electrons, thus converting the weak input signals into measurable currents. They typically consist of a photocathode and a downstream secondary electron multiplier (SEM) in an evacuated glass bulb. Their amplification factor is up to 108.
- Modern silicon photomultipliers (SiPM) are based on a single-photon avalanche diode (SPAD). These advanced avalanche photodiodes (APDs) use the photoelectric effect to generate charge carriers and the avalanche effect for internal amplification. SiPMs are optical sensors based on a silicon substrate that exhibit extremely high sensitivity and thus also enable the detection of weak light down to a single photon. In contrast to conventional photomultiplier tubes (PMTs), they require a significantly lower supply voltage. They are also used, for example, to detect individual particles in high-energy physics. Today, analog SiPMs make it possible to directly determine the photon rate from an output voltage. Digital SiPM chips meanwhile even offer the possibility of counting individual photons and simultaneously generating binary time stamps.
- Hybrid photodetectors (HPD, hybrid photomultiplier tubes, HPMT) detect very low light levels by combining the properties of photomultipliers and avalanche photodiodes. From a photocathode (e.g. made of gallium arsenide phosphide, GaAsP), which enables a high quantum yield, the photoelectrons are accelerated in a vacuum by means of an electric field and strike an avalanche photodiode (APD), so that the original photoelectron is multiplied extremely. Such detectors are able to determine the number of initially generated photoelectrons precisely due to their comparatively low statistical fluctuation of the final number of generated electrons, as long as the maximum recording capacity is not exceeded. The photocathode can usually be smaller than that in photomultipliers (PMTs).
- Daly detectors are high-performance ion counting devices with a very high dynamic range. They are therefore used to measure small samples and low-abundance isotopes and are frequently used in molecular beam experiments in combination with time-of-flight mass spectrometry. Their design allows the photomultiplier to be separated from the inside of the mass spectrometer. To do this, an aluminized cathode (a so-called “Daly knob” or a conversion dynode) is held at a very high negative potential in such a detector, so that ions are attracted to it so strongly that secondary electrons are emitted when they hit it. A high voltage is applied between the cathode and a scintillator, accelerating the electrons towards the scintillator, where they trigger photons that are directed through a glass window into a photomultiplier tube. The photomultiplier ultimately detects and counts the photons that pass through the glass window. The glass window only allows the photons to pass through, thus preventing contamination and increasing the photomultiplier's lifespan to up to five years.
- Channeltrons (Channel PhotoMultiplier, CPM) interact with electrons, ions and UV radiation and generate an electron avalanche from a primary particle. These work in a vacuum and generate massive secondary electron emissions at a high emission rate. The detectors are structures in glass or ceramic bodies that, depending on the design, have a horn-, circular-, sinusoidal- or spiral-shaped channel tube behind an input funnel. The inside of this tube is coated with an electron-emitting layer. When an electron hits this layer, it is accelerated by the applied high voltage and triggers an avalanche of secondary electrons in the subsequent collisions with the tube wall, which can be measured as voltage pulses. The curved shape of the channel tube ensures that the secondary electrons generated by a collision with the wall hit the wall multiple times, thus releasing further electrons. Their amplification factor is up to 108.
- Microchannel plate detectors (MCPs) are designed to detect electrons, ions or high-energy photons. They can be considered as an advancement of simple channeltrons and consist of a plate-shaped matrix of glass capillaries (between 10μm and 100μm inner diameter), which are coated on the inside with a semiconductor material. This layer on the capillary walls has electron-emitting properties, while the lead glass ensures high electrical resistance between the capillaries. Each of the capillaries acts as an electron multiplier during detection: an accelerating voltage is applied between the two metallized sides of the plate and the capillary is tilted slightly towards the plate axis so that incident electrons are sure to hit the wall of the respective capillary multiple times, repeatedly generating secondary electrons. These can then be recorded as measurable pulses using fast TDCs or ADCs. Microchannel plates operate with particularly low noise and, due to their matrix arrangement, can also provide spatial information if required. Their amplification factor is in the range of 104.
How are the data recorded in TOF mass spectrometry evaluated?
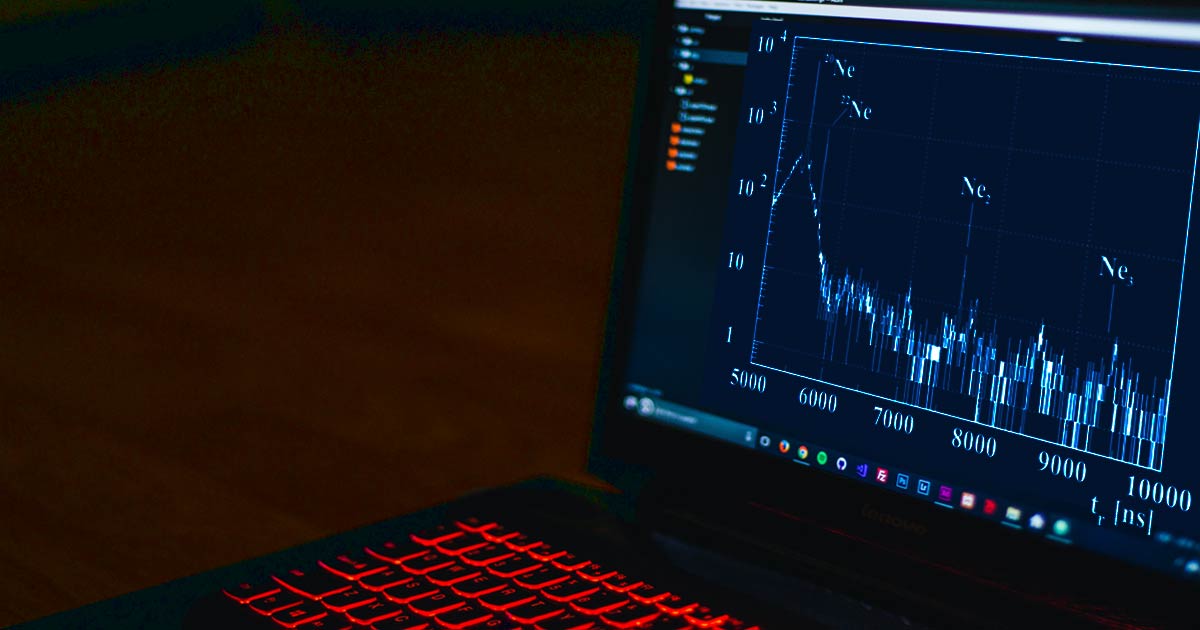
If an atom in a compound exists as naturally occurring isotopes, these will also be visible in the mass spectrum when using a high-quality ToF mass spectrometer, because the isotopes have different mass/charge ratios. This complexity offers additional possibilities for the determination of sample contents when evaluating the mass spectra. In order to identify sample contents, the following data are usually determined in a ToF mass spectrometer:
- The accurate mass of the ionized compound. In addition to the overall design of the mass spectrometer and the requirements that need to be met by the vacuum, accurate calibration of the device is vital for precise measurements. In many cases, this calibration is performed together with the actual sample measurement. For example, a calibration solution with known content is used for calibration.
- The relative abundance of isotopes. The relative abundance of isotopes can serve as a characteristic fingerprint for a given substance and also allows further information to be gathered about the content of the sample.
- The so-called isotope spacing. The isotope spacing which reflects the isotope distribution is also a characteristic feature of a substance.
The advantages of ToF mass spectrometry compared to other mass spectrometric methods:
- ToF-MS enables the simultaneous measurement of the mass-to-charge ratios of all ions almost without restriction on the mass range.
- Thanks to their enormous mass resolution, ToF measurements offer particularly precise detection options.
- In ToF measurements, no important information about sample contents is usually lost, so that even particularly complex mixtures can be characterized.
- Since even isotopes appear separately in the mass spectrum, ToF measurements facilitate the identification of analytes and thus the interpretation of the measurements.
Author: Uwe Thomaschky


